- Inicio
- kardia mobile 6l
- KardiaMobile 6L can be used to measure QT duration in COVID-19 patients - Cardiac Rhythm News
KardiaMobile 6L can be used to measure QT duration in COVID-19 patients - Cardiac Rhythm News
4.6 (757) · € 90.50 · En stock
KardiaMobile 6L, which it describes in a press release as the world’s only six lead personal ECG.

2021 ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals - Varma - 2021 - Annals of Noninvasive Electrocardiology - Wiley Online Library

RApid Throughput Screening for Asymptomatic COVID-19 Infection With an Electrocardiogram: A Prospective Observational Study - Mayo Clinic Proceedings: Digital Health

Manual QT interval measurement with a smartphone-operated single-lead ECG versus 12-lead ECG: a within-patient diagnostic validation study in primary care

Figure. Measuring the QT Interval in Different Clinical Scenarios

New FDA guidance allows use of KardiaMobile 6L to measure QTc in COVID-19 patients

Alivecor's Kardiamobile 6L joins COVID-19 fight to detect QT prolongation, 2020-03-23

Implementation of a fully remote randomized clinical trial with cardiac monitoring

Remote and wearable ECG devices with diagnostic abilities in adults: A state-of-the-science scoping review - Heart Rhythm
PDF) Feasibility of using KardiaMobile-L6 for QT interval monitoring during the early phase of the COVID-19 pandemic in critical care patients

Manual QT interval measurement with a smartphone-operated single-lead ECG versus 12-lead ECG: a within-patient diagnostic validation study in primary care
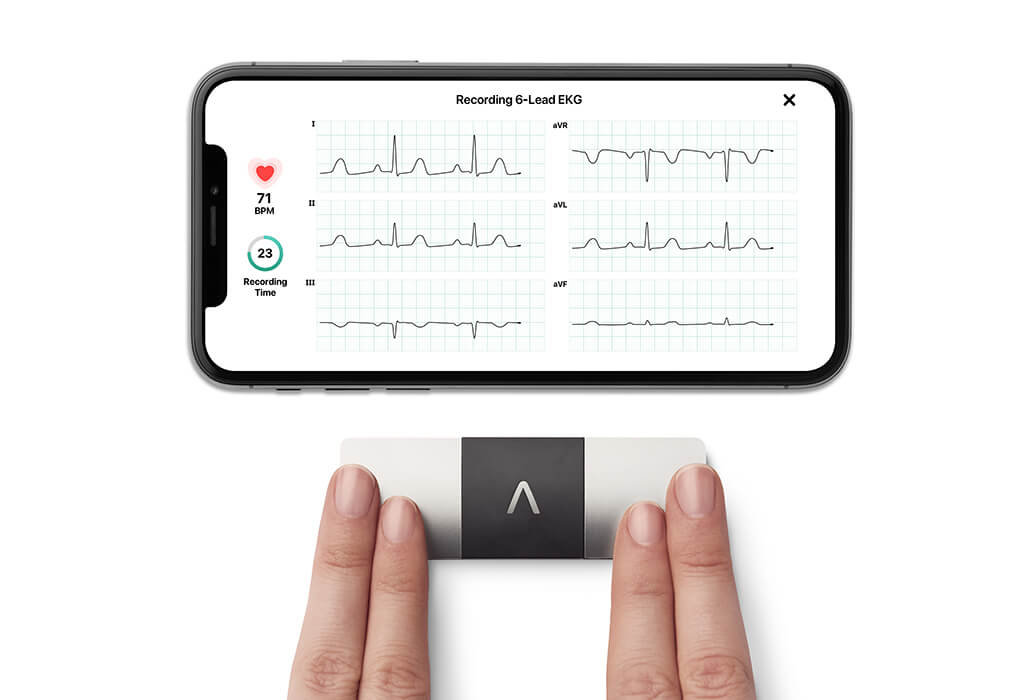
Keeping patients safe: antipsychotic medication monitoring using a pocket-sized six lead ECG - Lifescience Industry News

AliveCor scores QT prolongation clearance for its portable ECG to track dangerous drug side effects